On March 28, 2025, Abogen Biosciences announced that the Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) granted Investigational New Drug (IND) approval for the company’s lyophilized mRNA shingles vaccine.
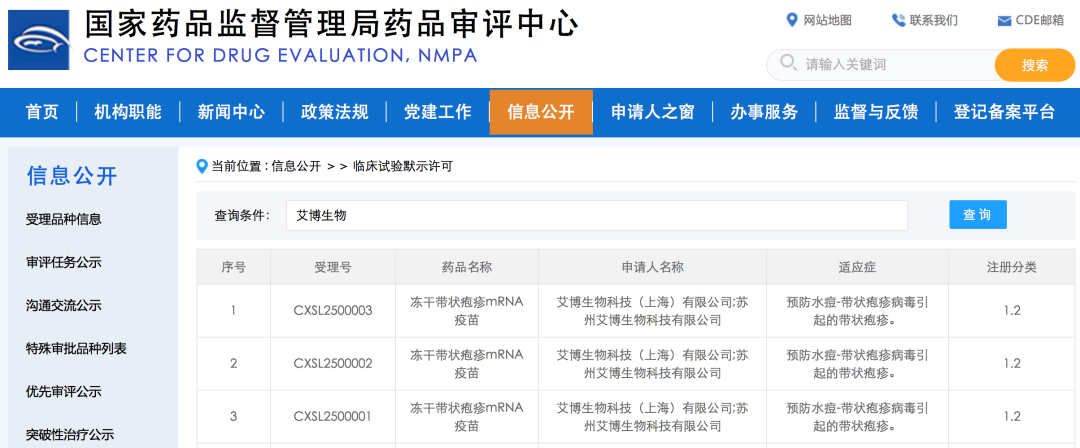
Following its recent approval of lyophilized mRNA vaccine targeting respiratory syncytial virus (RSV), this marks Abogen’s another vaccine candidate approved for clinical trials, once again highlighting the company's cutting-edge innovation platform. Additionally, leveraging the company's protein structure design platform, this newly approved vaccine features a novel, proprietary antigen design and has demonstrated strong immunogenicity, durable immune responses and good safety in preclinical studies.
Shingles, also known as herpes zoster, is caused by the reactivation of the varicella-zoster virus (VZV), characterized by skin lesions and neuropathic pain. While it can develop at any age, the incidence significantly increases with age, particularly in the elderly and immuno-compromised populations. In China, 99.5% of people over 50 are VZV carriers. Postherpetic neuralgia (PHN) is the most common complication of shingles, with severe and long-lasting pain. 30%–50% of PHN patients experience pain for over a year, and some cases persist for up to 10 years, greatly impacting the quality of life. In extreme cases, it can lead to drug dependence, despair, depression, or even suicide.
Vaccination is the most effective way to prevent shingles and PHN. Currently, available shingles vaccines worldwide include live-attenuated vaccines and recombinant vaccines, with mRNA-based VZV vaccines also under development. Compared to traditional technologies, mRNA vaccines can elicit a higher level of cellular immune response, offering a greater advantage in controlling virus reactivation.
The recent IND approvals of two lyophilized mRNA vaccines using the company's proprietary nucleoside base modification technology underscore the strength of Abogen's industry-leading innovation platform and its ability to consistently deliver high-quality products. These approvals also highlight the successful expansion of its Phase III-validated, internally developed mRNA technology platform from COVID-19 to other infectious diseases such as RSV and VZV.
Based on Abogen’s protein structure design platform, this newly IND approved mRNA Shingles Vaccine features a proprietary combination of mutation sites to engineer a structurally optimized gE protein as the antigen. Once the antigen protein sequence was determined, the company’s in-house RNA sequence design platform was then employed, incorporating codon optimization and UTR combinatorial screening to further enhance immunogenicity and druggability of the mRNA sequence. Preclinical data indicates that compared to the wild-type antigen, this vaccine induces higher immunogenicity, generating robust antibody and cell-mediated immune responses, with exceptional long-lasting immunity and a favorable safety profile.
In addition, this VZV vaccine incorporates Abogen’s unique nucleoside base modification technology and the only domestically developed, proprietary ionizable lipid validated in large-scale Phase III clinical trials. Unlike conventional liquid-frozen mRNA vaccines, it features an advanced lyophilized formulation, allowing for long-term storage at 2–8°C, thereby improving accessibility and convenience.
With its exceptional innovation technology platform, Abogen will continue to deliver high-quality products and achieve groundbreaking advancements in the fields of infectious diseases, immuno-oncology and protein replacement therapies.